THE UNIVERSITY OF BRITISH COLUMBIA
Science 1
Physics Assignment #
11:
THERMAL PHYSICS
Wed. 10 Jan. 2001 - finish by Wed. 17 Jan.
- 1.
- ``STAT - EC":
Consider the following simplified model of
a sort of stock market:
A given stock
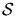 has a total of N shares on the market
for a fixed price
has a total of N shares on the market
for a fixed price
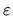 .
At a given time, n of these shares are
bought and the remaining N-n are unwanted.
Thus the net investment in
.
At a given time, n of these shares are
bought and the remaining N-n are unwanted.
Thus the net investment in 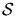 is
is
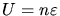 .
[Here
.
[Here
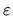 and U
are measured in monetary units, say dollars;
I have used the same notation as for energy
for reasons that will soon become evident.]
To keep things simple, we shall assume that
the price
and U
are measured in monetary units, say dollars;
I have used the same notation as for energy
for reasons that will soon become evident.]
To keep things simple, we shall assume that
the price
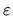 of a given stock
does not change. Further, let's make the
outrageous assumption that the stock market as a whole
is a priori equally likely to be found in
any one of the fully specified states accessible to it
-- i.e. that a given amount of capital is equally likely
to be distributed amongst the various stocks in any of
the possible ways that give the same total.1
of a given stock
does not change. Further, let's make the
outrageous assumption that the stock market as a whole
is a priori equally likely to be found in
any one of the fully specified states accessible to it
-- i.e. that a given amount of capital is equally likely
to be distributed amongst the various stocks in any of
the possible ways that give the same total.1
- (a)
- Invent a general definition for an economic analogue of
temperature
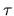 [measured in monetary units]
that has the desired predictive power:
that (given our starting assumptions)
capital will tend to flow spontaneously from stocks with
higher
[measured in monetary units]
that has the desired predictive power:
that (given our starting assumptions)
capital will tend to flow spontaneously from stocks with
higher 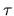 into others with lower
into others with lower 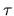 and will stop flowing between two stocks only when they
are in ``economic equilibrium" - i.e. when they have the
same ``economic temperature"
and will stop flowing between two stocks only when they
are in ``economic equilibrium" - i.e. when they have the
same ``economic temperature" 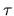 .
.
- (b)
- Now assume that the entire market is in ``economic
equilibrium" and is so much larger than any of its parts
that we may treat it as a ``capital reservoir"
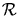 at an ``economic temperature" of
at an ``economic temperature" of 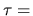 $100.
Consider one share of one stock, valued at
$100.
Consider one share of one stock, valued at
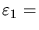 $200:
What is the probability that it will be bought at any given time?
$200:
What is the probability that it will be bought at any given time?
- (c)
- Assuming that
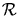 is also huge compared to
the entire offering of
N1 = 1000 shares of stock
is also huge compared to
the entire offering of
N1 = 1000 shares of stock
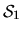 valued at
valued at
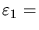 $200,
what is the expected total investment U1 in
$200,
what is the expected total investment U1 in
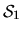 when
when 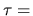 $100?
$100?
- (d)
- If the economic temperature drops to
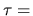 $50,
which stock will be likely to have the most capital U invested in it,
$50,
which stock will be likely to have the most capital U invested in it,
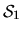 with
N1 = 1000 shares at
with
N1 = 1000 shares at
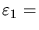 $200 per share
or
$200 per share
or
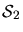 with
N2 = 1000 shares at
with
N2 = 1000 shares at
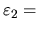 $100 per share?
$100 per share?
- 2.
- ORTHO- vs. PARA-HYDROGEN:
Molecular hydrogen, H2, consisting of two protons bound together
with two electrons, can form in either the ``singlet" state
called parahydrogen, in which the total spin
(intrinsic angular momentum) of the molecule is zero,
or in any one of three ``triplet" states of orthohydrogen,
in which the proton spins combine to make a total spin
of
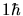 (the fundamental unit of angular momentum).
For this problem, all you need to know is that the three
triplet states are degenerate - i.e. they all
have the same energy relative to the singlet state,
namely
(the fundamental unit of angular momentum).
For this problem, all you need to know is that the three
triplet states are degenerate - i.e. they all
have the same energy relative to the singlet state,
namely
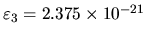 J.
(The energy
J.
(The energy
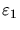 of the singlet state
can be taken to be zero, for reference.)
Assume that the spin degrees of freedom of the H2
molecules are unaffected by,
but are in thermal equilibrium with,
all their other degrees of freedom
(like translational, rotational or vibrational).
In this case, what fraction f3 of H2 molecules
will be found (on average) in ortho states
of the singlet state
can be taken to be zero, for reference.)
Assume that the spin degrees of freedom of the H2
molecules are unaffected by,
but are in thermal equilibrium with,
all their other degrees of freedom
(like translational, rotational or vibrational).
In this case, what fraction f3 of H2 molecules
will be found (on average) in ortho states
- (a)
- at room temperature (300 K)?
- (b)
- at the boiling point of liquid nitrogen
at atmospheric pressure (77 K)?
- (c)
- at the freezing point of molecular hydrogen
at atmospheric pressure (14 K)?
Jess H. Brewer
2001-01-28
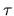 [measured in monetary units]
that has the desired predictive power:
that (given our starting assumptions)
capital will tend to flow spontaneously from stocks with
higher
[measured in monetary units]
that has the desired predictive power:
that (given our starting assumptions)
capital will tend to flow spontaneously from stocks with
higher 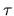 into others with lower
into others with lower 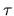 and will stop flowing between two stocks only when they
are in ``economic equilibrium" - i.e. when they have the
same ``economic temperature"
and will stop flowing between two stocks only when they
are in ``economic equilibrium" - i.e. when they have the
same ``economic temperature" 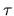 .
.
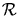 at an ``economic temperature" of
at an ``economic temperature" of 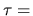 $100.
Consider one share of one stock, valued at
$100.
Consider one share of one stock, valued at
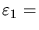 $200:
What is the probability that it will be bought at any given time?
$200:
What is the probability that it will be bought at any given time?
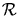 is also huge compared to
the entire offering of
N1 = 1000 shares of stock
is also huge compared to
the entire offering of
N1 = 1000 shares of stock
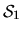 valued at
valued at
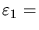 $200,
what is the expected total investment U1 in
$200,
what is the expected total investment U1 in
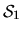 when
when 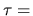 $100?
$100?
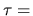 $50,
which stock will be likely to have the most capital U invested in it,
$50,
which stock will be likely to have the most capital U invested in it,
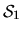 with
N1 = 1000 shares at
with
N1 = 1000 shares at
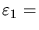 $200 per share
or
$200 per share
or
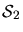 with
N2 = 1000 shares at
with
N2 = 1000 shares at
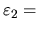 $100 per share?
$100 per share?