Next: Magnetic Interactions
Up: Orbital Angular Momentum
Previous: Orbital Angular Momentum
One of Niels Bohr's main contributions to Physics
was his assertion (backed up by experiment) that
angular momentum is quantized -
it can only occur in integer multiples of 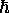 .
Erwin Schr´'odinger showed why this was true for
the wave functions of the hydrogen atom, but by that
time Bohr's principle had been elevated to an empirical
``law'' of Physics that went well beyond the realm of atoms.
Schr´'odinger also showed the peculiar nature of the
quantization of
.
Erwin Schr´'odinger showed why this was true for
the wave functions of the hydrogen atom, but by that
time Bohr's principle had been elevated to an empirical
``law'' of Physics that went well beyond the realm of atoms.
Schr´'odinger also showed the peculiar nature of the
quantization of  : first, its magnitude obeys
: first, its magnitude obeys
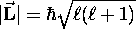 where
where
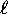 can only have integer values from zero to
can only have integer values from zero to
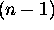 , n being the principle quantum number
for which
, n being the principle quantum number
for which 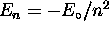 in the case of hydrogen;
second, its projection onto the z axis obeys
in the case of hydrogen;
second, its projection onto the z axis obeys
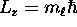 where
where 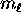 can take on
only integer values from
can take on
only integer values from 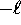 to
to 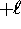 .
Note that Bohr's original prescription for angular
momentum quantization (integer multiples of
.
Note that Bohr's original prescription for angular
momentum quantization (integer multiples of 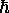 )
is actually applicable to the z component
of
)
is actually applicable to the z component
of  - its projection
onto the z
quantization axis, which is chosen arbitrarily
unless there is a magnetic field applied, in which case
- its projection
onto the z
quantization axis, which is chosen arbitrarily
unless there is a magnetic field applied, in which case
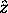 is always chosen along the field,
is always chosen along the field,
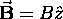 .
.
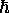 .
Erwin Schr´'odinger showed why this was true for
the wave functions of the hydrogen atom, but by that
time Bohr's principle had been elevated to an empirical
``law'' of Physics that went well beyond the realm of atoms.
Schr´'odinger also showed the peculiar nature of the
quantization of
.
Erwin Schr´'odinger showed why this was true for
the wave functions of the hydrogen atom, but by that
time Bohr's principle had been elevated to an empirical
``law'' of Physics that went well beyond the realm of atoms.
Schr´'odinger also showed the peculiar nature of the
quantization of  : first, its magnitude obeys
: first, its magnitude obeys
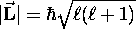 where
where
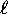 can only have integer values from zero to
can only have integer values from zero to
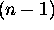 , n being the principle quantum number
for which
, n being the principle quantum number
for which 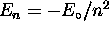 in the case of hydrogen;
second, its projection onto the z axis obeys
in the case of hydrogen;
second, its projection onto the z axis obeys
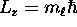 where
where 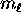 can take on
only integer values from
can take on
only integer values from 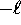 to
to 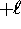 .
Note that Bohr's original prescription for angular
momentum quantization (integer multiples of
.
Note that Bohr's original prescription for angular
momentum quantization (integer multiples of 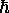 )
is actually applicable to the z component
of
)
is actually applicable to the z component
of  - its projection
onto the z
quantization axis, which is chosen arbitrarily
unless there is a magnetic field applied, in which case
- its projection
onto the z
quantization axis, which is chosen arbitrarily
unless there is a magnetic field applied, in which case
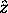 is always chosen along the field,
is always chosen along the field,
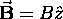 .
.