BELIEVE ME NOT! -  - A SKEPTIC's GUIDE
- A SKEPTIC's GUIDE


Next: Protection Against Radiation
Up: Radiation Hazards
Previous: Sources of Radiation
The information given above would seem to indicate that
medical X-rays were the worst radiation hazard around,
except for natural sources we can't do much about.
Unfortunately this is a distortion based on the difficulty
of measuring the most dangerous kind of radiation:
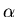 -emitting radionuclides (radioactive isotopes).
Many heavy elements have isotopes which naturally
fission into lighter elements plus a helium nucleus,
with the latter being emitted with a substantial
kinetic energy as an alpha "ray." The range of
most
-emitting radionuclides (radioactive isotopes).
Many heavy elements have isotopes which naturally
fission into lighter elements plus a helium nucleus,
with the latter being emitted with a substantial
kinetic energy as an alpha "ray." The range of
most 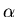 particles is only a few cm in air
and less than a mm in tissue, so the damage they cause
is localized. While this may be reassuring when the
isotopes are at arm's length, it can be bad news
if you have breathed them into your lungs or
swallowed them so that they can collect in your bones,
where they can do the most damage! Since there is
such a wide variety of radioactive elements with
assorted chemical properties, it is wise to be aware
of the specific hazards associated with each.
I have neither the expertise nor the space to
provide a comprehensive survey here, but I can
mention a few of the most common culprits.
particles is only a few cm in air
and less than a mm in tissue, so the damage they cause
is localized. While this may be reassuring when the
isotopes are at arm's length, it can be bad news
if you have breathed them into your lungs or
swallowed them so that they can collect in your bones,
where they can do the most damage! Since there is
such a wide variety of radioactive elements with
assorted chemical properties, it is wise to be aware
of the specific hazards associated with each.
I have neither the expertise nor the space to
provide a comprehensive survey here, but I can
mention a few of the most common culprits.
- Radon: All rock contains
some amount of naturally occurring radium
which gradually decays, releasing the chemically
inert noble gas radon. Radon in turn is a
radioactive element which decays by emitting a rather
low energy
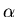 particle that is quite difficult
to detect since it has such a short range it can't
penetrate the window of a typical Geiger counter.
Thus until recently there was little known about
radon in our environment, even though it is generally
believed that Madame Curie died from
exposure to radon emitted by the radium upon which
she performed her famous experiments.
It is now felt by many that radon is the most
widespread and dangerous of all radiation hazards,
because it accumulates in the air of any building
made of rock, brick or concrete (especially those
with closed circulation air conditioning!)
and thence in the lungs of the people breathing
that air. Lungs in fact make a superb filter
for the radioactive byproducts of radon, so that
one of the most effective radon detection schemes
is to measure the radioactivity of the people
who live in high-radon environments.
In the lung tissue, the short-ranged
particle that is quite difficult
to detect since it has such a short range it can't
penetrate the window of a typical Geiger counter.
Thus until recently there was little known about
radon in our environment, even though it is generally
believed that Madame Curie died from
exposure to radon emitted by the radium upon which
she performed her famous experiments.
It is now felt by many that radon is the most
widespread and dangerous of all radiation hazards,
because it accumulates in the air of any building
made of rock, brick or concrete (especially those
with closed circulation air conditioning!)
and thence in the lungs of the people breathing
that air. Lungs in fact make a superb filter
for the radioactive byproducts of radon, so that
one of the most effective radon detection schemes
is to measure the radioactivity of the people
who live in high-radon environments.
In the lung tissue, the short-ranged 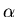 particles
expend all their energy where it does the most harm,
raising the incidence of lung disease and cancer.
Rocks from different regions have a tremendous range
of radium content, so that a stone house may be
perfectly safe in one city and hazardous in another.14
particles
expend all their energy where it does the most harm,
raising the incidence of lung disease and cancer.
Rocks from different regions have a tremendous range
of radium content, so that a stone house may be
perfectly safe in one city and hazardous in another.14
- Potassium and Carbon:
Radioisotopes of potassium and carbon are continually
created in the atmosphere by cosmic ray bombardment;
these isotopes build up to a constant level in all
living tissues, only to decay away in a few thousand
years after death. This means that the most radioactive
component in your household is probably you!15
It also provides a handy method
of estimating the time since formerly living matter
was alive (14C and potassium-argon dating).
- Man-made Radionuclides:
There are too many of these to make a comprehensive
list here.16
The most famous is plutonium, 239Pu,
the stuff of which fission bombs are made. Plutonium
is both a deadly chemical poison and a nasty radioisotope.
If a miniscule grain is caught in your lungs or other
tissues, it may not do much damage to your body as a whole,
but it exposes the tissue immediately around it to a huge
dose of radiation, drastically increasing the likelihood
of cancer in that tissue. Cancer is just as deadly
no matter where it begins, which makes the ingestion of
radionuclides the worst possible sort of radiation hazard.
It is important to note that the food chain may
serve to concentrate "harmless" levels of radionuclides
in (e.g.) sea water to a level which is worthy of our
concern. Were it not for this effect, and the fact that
the waste products of nuclear fission include a large
variety of radionuclides with various chemical properties
that naturally occurring isotopes do not exhibit,
it would be a sensible strategy to dispose of radioactive
waste by diluting it and spreading it far and wide in the
oceans - since the net radioactivity of reactor fuel
actually decreases in the process of digging up
the uranium, burning it in a reactor and storing the
spent fuel rods for 10 years until the short-lived isotopes
decay away. Because of the biological concentration effect,
however, it is wiser to seek safe long-term containments
for radioactive waste.


Next: Protection Against Radiation
Up: Radiation Hazards
Previous: Sources of Radiation
Jess H. Brewer -
Last modified: Mon Nov 23 13:47:23 PST 2015
 - A SKEPTIC's GUIDE
- A SKEPTIC's GUIDE  - A SKEPTIC's GUIDE
- A SKEPTIC's GUIDE